Search
Neurosurgeon Focused on Care of the Spine and Brain
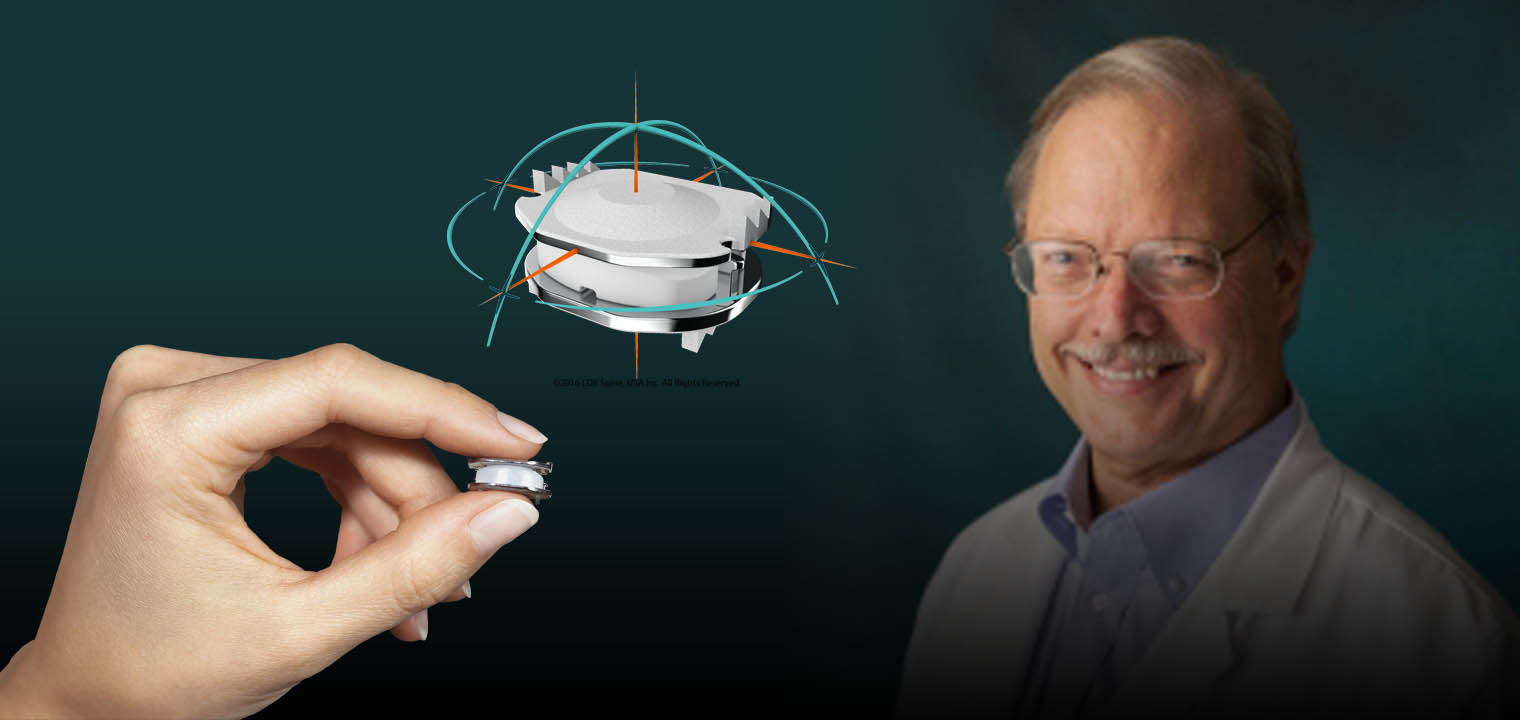
Dr. Gaede has had additional, post-residency training and experience in the use of CyberKnife, Minimally Invasive Surgery, Stereotactic brain surgery, spinal instrumentation and artificial disc surgery. He has demonstrated clinical research interests. He was named one of thirty U.S. Principal Investigators for the FDA trial of Deep Brain Stimulation therapy for Parkinson's disease and Essential Tremor. He was also a Principal Investigator for the FDA’s Mobi-C artificial disc trials. The Mobi-C was the first and only artificial disc in the U.S. FDA approved to treat more than one level of the cervical spine. It received FDA approval in August 2013. Dr. Gaede also has extensive experience in Hospital Administration and has held many leadership positions including the presidency of Tulsa Spine and Specialty Hospital from 2002 to the present. He has been instrumental in the development and growth of TSSH, which has blossomed into a center of excellence, attracting patients for both its unrivaled patient satisfaction surveys and its survey-proven quality.
Steven Gaede Curriculum Vitae
A PDF copy of Dr. Gaede's CV is available for download and viewing. gaedecv.pdf
Website Design by KW Consulting Plus.
f (918) 744-4246